Press Releases
FILTER BY
- All
- Aerospace & Defense
- Best Practices Awards
- Business & Financial Services
- Chemicals, Materials & Nutrition
- Energy & Environment
- Healthcare
- Industrial
- Information & Communications Technology
- Manufacturing
- Mobility
- TechVision
- White Papers
Vodafone Earns Frost & Sullivan’s 2024 European Company of the Year Award for Redefining Water Management Technology across Europe
Vodafone’s transformative initiatives and unwavering dedication to innovation have reshaped the European water management landscape, addressing critical challenges and driving sustainable outcomes.
Scaling Up AI Deployments: Harnessing Opportunities Sparked by Growing AI Maturity
By Frost & Sullivan
Apptio Earns Frost & Sullivan’s 2024 North American New Product Innovation Award for Streamlining Enterprise Agility with Targetprocess
Apptio pioneers a transformative approach to enterprise agility with its Targetprocess platform, simplifying the integration of scaled agile framework (SAFe 6.0) standards into businesses.
Teleperformance Recognized with Frost & Sullivan’s 2024 Global Company of the Year Award for Enhancing Agent Engagement with the Latest AI Technologies
Teleperformance sets a new benchmark in the customer experience industry by combining sophisticated technologies and human empathy to engage employees in support of thecustomer journey.
Broadcom Earns Frost & Sullivan’s 2024 Global Company of the Year Award for Delivering Reliable and Flexible Hybrid Cloud Management Solutions
Broadcom’s VMware software leads the industry with its innovative approach to hybrid cloud management, providing businesses with unparalleled visibility, automation, and control over diverse IT environments.
JLL Asia Pacific Awarded Frost & Sullivan’s 2023 APAC Company of the Year Award for Delivering a Broad Product Portfolio That Meets Industry Demands and Customer Expectations
Jones Lang LaSalle Asia Pacific (JLL) addresses unmet market needs with its strong leadership vision that incorporates unrivaled customer-centric strategies and outstanding strategy implementation.
Ecosystem Collaborations will be Pivotal if Kick Scooter Sharing Services Market is to Overcome Safety Concerns
Almost a year to date in April 2023, citing reckless driving, haphazard parking, and safety issues, nearly 90% of Parisiennes voted to ban e-scooter rentals in the city. By September 1, the 15,000 strong e-scooter fleet headlined by companies like Lime, Dott and Tier...
Ottopia Selected as the Global Market Leader of Automotive Teleoperation by Frost & Sullivan
Ottopia builds and provides tele-driving technology to enable luxury, convenience, efficiency, and innovation in the mobility ecosystem, revolutionizing the automotive industry.
TOP STRATEGIC IMPERATIVES SHAPING THE MATERIALS INDUSTRY
By Frost & Sullivan
iSono Health Applauded by Frost & Sullivan for Ensuring Patient Comfort and Safety and Providing Quality Imaging and Diagnosis with Its iSono Health ATUSA
iSono Health AI ABUS ATUSA enables precise and prompt screening, eliminates delays and the need for trained technicians, provides cost-effective imaging, and improves patient outcomes significantly.
Are You Collaborating with the Shared Mobility Startup Ecosystem for Sustained Growth?
By Frost & Sullivan
Burgeoning Freight and Logistics Demands to Fuel Recovery of China’s Commercial Truck Industry
Truck electrification emerges as a major trend with electric powertrains set to reach 1 million units in 2030 on the back of technological improvements and government support. Commercial truck sales in China plunged from 3.7 million units in 2021 to 2.4 million units...
Are You Optimizing Your Growth Strategy to Leverage Key Opportunities in the Machines Industry?
By Frost & Sullivan
Singtel Awarded Frost & Sullivan’s 2023 Singapore Company of the Year Award for Its Strong Industry Leadership Position in Cybersecurity Services
Singtel is reputable in the cybersecurity services industry, driven by its robust investment in innovation, unwavering commitment to expanding cybersecurity service offerings, comprehensive security solutions, and premium support.
AeC Recognized by Frost & Sullivan for Leading the Customer Relationship Industry in Brazil
AeC is the largest customer relationship company in Brazil and its reliable and customized customer care solutions help businesses create seamless end-user experiences.
STT GDC India Earns Frost & Sullivan’s 2023 Indian Company of the Year Award for Its Cutting-edge Innovation and Ethical and Eco-responsible Business Practices
ST Telemedia Global Data Centers India (STT GDC India) provides cost-effective, best-in-class solutions with state-of-the-art technology and sustainable practices.
GenieMD Awarded Frost & Sullivan’s 2024 Company of the Year Award for Pioneering Virtual Chronic Disease Management Solutions Through Its Unified Virtual Care Platform
GenieMD offers a comprehensive virtual care platform, integrating telehealth, remote patient monitoring, chronic care management, and AI-driven insights to impact the treatment and management of chronic diseases.
Are You Leveraging the Drug Discovery and Early Development Outsourcing Services Ecosystem to Accelerate Growth?
By Frost & Sullivan
Cutting-edge Technologies Catalyze the Global Commercial Aircraft Aerostructures Market Growth
With sustainability as a key tool, the commercial aerospace industry is enhanced by innovative technologies
New Satellite Design Advances Fuel Market Growth for Satellite Propulsion Solutions
Satellite constellations generate ongoing demand for propulsion systems, according to Frost & Sullivan
Global Maritime SATCOM Services Growth to be Boosted by Smart Shipping
Rising demand for broadband connectivity among customers and crew members drives several segments of the maritime industry to adopt SATCOM services
Landing Gear System Market on a Growth Trajectory as Regional Connectivity Surges
The adoption of ultra-low-cost carrier business model expedites the global commercial aircraft landing gear system industry, says Frost & Sullivan
Global Aircraft Tire Market Growth Driven by the Recovery of the Aviation Industry
Growth potential in commercial flights will propel the demand for aircraft tires, with sustainability as a key investment area among vendors, says Frost & Sullivan
Global Ground Station Services Market to be Transformed by Innovative Business Models
Emerging business models help users cost-effectively schedule their data exchange tasks with their respective satellites, says Frost & Sullivan
How Can Airports Reach Sustainability Goals by Investing in Waste Management
Green efforts and focus on waste management will increase as air passenger traffic recovers, says Frost & Sullivan.
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Innovation in Surveillance Technologies Ignites Global Surveillance Solutions Market Growth
Industry verticals need to address security concerns and acquire business intelligence data, says Frost & Sullivan.
Digitalization and Innovative Business Models Key to Transformational Growth in APAC, Finds Frost & Sullivan
With digital transformation and new technologies already disrupting multiple industries, organizations must innovate for the future to create value and drive better business outcomes.
Frost & Sullivan and TERI’s Sustainability 4.0 Awards 2021 Honor Companies Embedding Sustainability with Economic Value Creation
At the 12th edition, 23 awards were presented to companies for their exemplary performance in sustainability
Improvements in Flight Operations Expediting Global Commercial Avionics Market Growth
Regulatory mandates and new aircraft purchases inflate the global demand for avionics systems, says Frost & Sullivan.
Frost & Sullivan Marks its 60th Anniversary with a Renewed Focus on Enhanced Core Competencies and Streamlined Customer Experience
The company’s new digital platform will provide an expansion of data analytics to continually improve accuracy and service delivery
Global Aviation Satcom Market to Take Off as Airlines Offer Better Passenger Experience, Says Frost & Sullivan
The global aviation Satcom market is estimated to reach $730.4 million by 2030 from $527.2 million in 2020.
Frost & Sullivan Reveals the Top 10 Global Economic Trends Shaping the Growth Prospects in 2021 and 2022
Discover how the 2022 economic growth outlook is set to evolve globally and potential factors impacting businesses in our upcoming growth opportunity briefing.
Frost & Sullivan Reveals the Growth Opportunities in the Urban Air Mobility Industry
In this webinar, learn about the existing Urban Air Mobility (UAM) value chain, state of regulations, and likely roadmap to allow UAM operations globally. In addition, understand the links between the evolving technology supplier landscape and major UAM integrators.
Global Airline Digitalization Gains Traction, Thanks to Digital Technologies and Data Analytics
Asia-Pacific is expected to remain the largest revenue contributor to the airline digitalization market in 2030, while North America will be the fastest-growing region.
Frost & Sullivan Analyzes the Future of Digital Identity Management and Value Chain Compression
Join Frost & Sullivan’s webinar highlighting the impact of digital identity management and value chain compression on societies and industries on July 13th, 11 a.m. (EDT).
Vodafone Earns Frost & Sullivan’s 2024 European Company of the Year Award for Redefining Water Management Technology across Europe
Vodafone’s transformative initiatives and unwavering dedication to innovation have reshaped the European water management landscape, addressing critical challenges and driving sustainable outcomes.
Apptio Earns Frost & Sullivan’s 2024 North American New Product Innovation Award for Streamlining Enterprise Agility with Targetprocess
Apptio pioneers a transformative approach to enterprise agility with its Targetprocess platform, simplifying the integration of scaled agile framework (SAFe 6.0) standards into businesses.
Teleperformance Recognized with Frost & Sullivan’s 2024 Global Company of the Year Award for Enhancing Agent Engagement with the Latest AI Technologies
Teleperformance sets a new benchmark in the customer experience industry by combining sophisticated technologies and human empathy to engage employees in support of thecustomer journey.
Broadcom Earns Frost & Sullivan’s 2024 Global Company of the Year Award for Delivering Reliable and Flexible Hybrid Cloud Management Solutions
Broadcom’s VMware software leads the industry with its innovative approach to hybrid cloud management, providing businesses with unparalleled visibility, automation, and control over diverse IT environments.
JLL Asia Pacific Awarded Frost & Sullivan’s 2023 APAC Company of the Year Award for Delivering a Broad Product Portfolio That Meets Industry Demands and Customer Expectations
Jones Lang LaSalle Asia Pacific (JLL) addresses unmet market needs with its strong leadership vision that incorporates unrivaled customer-centric strategies and outstanding strategy implementation.
Ottopia Selected as the Global Market Leader of Automotive Teleoperation by Frost & Sullivan
Ottopia builds and provides tele-driving technology to enable luxury, convenience, efficiency, and innovation in the mobility ecosystem, revolutionizing the automotive industry.
iSono Health Applauded by Frost & Sullivan for Ensuring Patient Comfort and Safety and Providing Quality Imaging and Diagnosis with Its iSono Health ATUSA
iSono Health AI ABUS ATUSA enables precise and prompt screening, eliminates delays and the need for trained technicians, provides cost-effective imaging, and improves patient outcomes significantly.
Singtel Awarded Frost & Sullivan’s 2023 Singapore Company of the Year Award for Its Strong Industry Leadership Position in Cybersecurity Services
Singtel is reputable in the cybersecurity services industry, driven by its robust investment in innovation, unwavering commitment to expanding cybersecurity service offerings, comprehensive security solutions, and premium support.
AeC Recognized by Frost & Sullivan for Leading the Customer Relationship Industry in Brazil
AeC is the largest customer relationship company in Brazil and its reliable and customized customer care solutions help businesses create seamless end-user experiences.
STT GDC India Earns Frost & Sullivan’s 2023 Indian Company of the Year Award for Its Cutting-edge Innovation and Ethical and Eco-responsible Business Practices
ST Telemedia Global Data Centers India (STT GDC India) provides cost-effective, best-in-class solutions with state-of-the-art technology and sustainable practices.
GenieMD Awarded Frost & Sullivan’s 2024 Company of the Year Award for Pioneering Virtual Chronic Disease Management Solutions Through Its Unified Virtual Care Platform
GenieMD offers a comprehensive virtual care platform, integrating telehealth, remote patient monitoring, chronic care management, and AI-driven insights to impact the treatment and management of chronic diseases.
Intradiem Awarded Frost & Sullivan’s 2024 Global New Product Innovation Award for Reducing Contact Center Agent Burnout with Its Advanced AI Solutions
The Intradiem AI-powered Burnout and Attrition Indicator dramatically enhances agent performance with an impressive 80% accuracy in predicting staff turnover
Tata Communications Recognized by Frost & Sullivan for Excellence in Next-gen Connectivity and Unified Communications
Commtech player receives four Indian Service Provider Company of the Year Awards for its enterprise solutions
Sectigo Applauded by Frost & Sullivan for Innovations in Automated Certificate Lifecycle Management and Offering Superior Customer Value with Sectigo Certificate Manager
Certificate Manager provides full visibility into cryptographic assets, reduces human error, and costs, and delivers operational efficiencies and compliance with industry and regulatory standards
Secureworks Earns Frost & Sullivan Competitive Strategy Leadership Award in the Global Extended Detection and Response Market
“Innovation powerhouse” Secureworks applauded for its innovation and customer-first approach which makes them “a force to be reckoned with in the XDR market.”
Forcepoint Awarded Frost & Sullivan’s 2023 Global Company of the Year Award for Pioneering the Data Loss Prevention Industry
Forcepoint revolutionizes data-first cybersecurity with its ability to empower people to work anywhere by securing data everywhere, enabling businesses of all sizes to safeguard sensitive data successfully across various environments.
Spectrum Enterprise Recognized with Frost & Sullivan’s 2024 Company of the Year Award for Its Fiber Ethernet Services
Spectrum Enterprise stands out in the US Ethernet services industry with its high-performance network and connectivity services that cater to the unique needs of numerous industries
Teleperformance Applauded by Frost & Sullivan for Helping Clients Optimize and Transform Their CX for Simpler, Faster, and Safer Interactions
Teleperformance develops solutions that meet clients’ increasingly complex needs, in terms of individual safety, data, and systems security, to maintain its leadership position.
Halal Economy Thrives as Product Demand from Muslims and non-Muslim Nations Surges
The buoyant market for the global halal economy is expected to reach $4.96 trillion by 2030, says Frost & Sullivan
Businesses Winning Women’s Hearts to Thrive: 9 Mega Trends Leading the Way in the Sheconomy
With a significant rise in women’s consumer spending, businesses across industries are turning toward AI-enabled solutions to better understand female clients, says Frost & Sullivan.
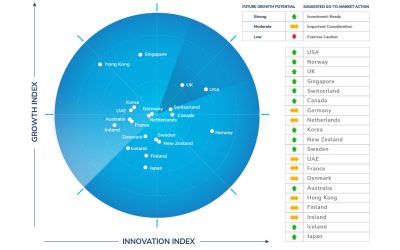
Top 3 Countries for Future Growth Potential: The United States, Norway, and the United Kingdom
Based on macroeconomic growth indicators and citizen-centricity, the United States, Norway, and the United Kingdom have been ranked the top three countries, demonstrating high future growth potential, according to the 2022 edition of Frost & Sullivan’s Frost Radar™ for Economic Development.
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Frost & Sullivan Reveals Strategic Growth Opportunities Amidst Global Economic Recovery in 2022
Think Tank will explore how sustainability, digitalization and economic trends across regions are shaping regional and industry outlook
Tailored Digital Platforms Help Banks Enhance the Customer Experience throughout the Customer Journey
Banks use technology partners to spread risks while raising customer service levels, finds Frost & Sullivan
Financial Service Companies Look to Elevate Security by Partnering with Cloud Experts Offering Integrated Solutions
The financial services industry is amongst the earliest adopters of cloud and other new digital technologies. However, due to security risks, the road to modernisation has its challenges. Can the right solution provider ensure successful outcomes?
Frost & Sullivan Reveals the Top 10 Global Economic Trends Shaping the Growth Prospects in 2021 and 2022
Discover how the 2022 economic growth outlook is set to evolve globally and potential factors impacting businesses in our upcoming growth opportunity briefing.
Frost & Sullivan Analyzes the Future of Digital Identity Management and Value Chain Compression
Join Frost & Sullivan’s webinar highlighting the impact of digital identity management and value chain compression on societies and industries on July 13th, 11 a.m. (EDT).
Frost & Sullivan Commemorates Juneteenth as a Federal Holiday
The DEI Alliance at Frost & Sullivan honors the latest holiday addition with educational and celebratory resources.
Frost & Sullivan Announces New Vice President of Human Resources for Middle East, Africa and South Asia
She will be responsible for leading all human resources functions in the region and ensuring that the company continues to build a culture that attracts, engages, and develops the best teams.
Frost & Sullivan Unveils Strategic Opportunities in Digital Upskilling Shaping the Future of Work and Crowd Analytics
Various transformative trends and emerging opportunities are shaping the future of work and powering business and operational intelligence, opening up new avenues in digitalization
Frost & Sullivan Analyzes the Mega Trends Transforming the United States Through 2030
Millennials will remain the largest age group the US has ever seen (26.6% in 2030), ushering in a dramatic national shift as this population moves into leadership positions, family roles, and suburban living.
Global Companies Turn to Cloud-Based, Unified Solutions to Address Rising Demands from Investors, Regulators, and Internal Stakeholders
Frost & Sullivan’s white paper identifies factors driving the uptake of cloud-based reporting solutions and the advantages they bring.
Frost & Sullivan Experts to Analyze Economic Outlook of a Post-pandemic 2021
Frost & Sullivan outlines top 2021 predictions and growth opportunities expected to transform the global economic landscape.
8 Global Shifts for 2021 Reshaping Industries, Governments and Society
Changes in user behavior patterns will trigger major changes in consumption and business models.
5 Growth Opportunities to Seize in the Fintech Industry in 2021
Discover key trends and strategic recommendations in cybersecurity, big data, partnerships, digital banks, and personalization.
Frost & Sullivan Evaluates the Future of Corporate Transactions Reshaped by COVID-19
Frost & Sullivan invites you to the upcoming webinar, “Prepare for the Future of Corporate Transactions,” on Nov. 18, 2020 at 2 p.m. GMT / 9 a.m. EST. Join Frost & Sullivan Business & Financial Services experts George Galanopoulos, Sanjay Singh, and Akhil Bajaj, to discover the key fundamental changes brought about by COVID-19 in the M&A process and how this can be optimized in the new normal.
Global Transformation: The Dynamics of Mergers & Acquisitions in the Packaging Industry
The packaging industry is filled with instances of mergers & acquisitions (M&A) as there is limited room for the industry to grow on its own. Its total dependence on end markets compels players to seek M&A activities to expand their capabilities and stay competitive.
Supply Chain Visibility to Ensure Humanitarian Assistance for All in Need
NEC focuses efforts on leveraging digital technologies to contribute to resolving supply chain visibility problems
Advantages of Hot Melt Adhesives (HMAs) Drive the Industry’s Growth
The global HMAs market is expected to garner $13.81 billion in revenue by 2028, registering growth at a CAGR of 6.3%, says Frost & Sullivan.
Global Cannabidiol Legalization Trend to Shape the CBD Market’s Future Growth
The global market for CBD will likely reach $4.09 billion by 2030, expanding at a CAGR of 9.5%, says Frost & Sullivan
Collaboration Between Drug and Medical Food Manufacturers to Boost Market Growth
Frost & Sullivan says the prevalence of lifestyle diseases and geriatric population drives demand for medical food globally.
Growth of Sustainable Farming Practices Drives the Agricultural Biologicals Market
The increasing need for crop protection products and product awareness will expand the biopesticides and biostimulants segments, says Frost & Sullivan.
The Global Aquaculture Market Witnesses Growth as the Demand for Protein Surges
The industry is a sustainable means to supply and feed the global population with environment-friendly protein products through smart farming, says Frost & Sullivan.
The Outbreak of COVID-19 Unlocked the Demand for Antimicrobial Technologies
Innovations triggered by the pandemic are pushing industries to adopt long-lasting antimicrobial technology, says Frost & Sullivan
Halal Economy Thrives as Product Demand from Muslims and non-Muslim Nations Surges
The buoyant market for the global halal economy is expected to reach $4.96 trillion by 2030, says Frost & Sullivan
Will the flooring materials market continue catering to its consumers with technological innovations?
The flooring materials market is poised to grow at a CAGR of 5-6%.
Global Automotive Plastics Market Boosted by the Need to Minimize CO2 Emissions
The global automotive sector will inflate the demand for sustainability targets to minimize CO2 emissions in the next 3–5 years, says Frost & Sullivan.
Spike in Geriatric Population Boosts Bone and Joint Health Ingredients Market
Manufacturers should focus on higher bioavailability ingredients and innovation says, Frost & Sullivan.
How DSM Merged with Firmenich: When Capabilities Complement each other
The complementary capabilities of the two companies are the biggest competitive advantage and will offer higher innovation and growth acceleration opportunity in the Nutrition, Health, & Beauty Space
How Global Healthcare Spending and Regulations Boost the Surgical Gloves Market
Demand for environmentally friendly biodegradable surgical gloves unlocks new opportunities for market participants, says Frost & Sullivan.
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Digitalization and Innovative Business Models Key to Transformational Growth in APAC, Finds Frost & Sullivan
With digital transformation and new technologies already disrupting multiple industries, organizations must innovate for the future to create value and drive better business outcomes.
Frost & Sullivan and TERI’s Sustainability 4.0 Awards 2021 Honor Companies Embedding Sustainability with Economic Value Creation
At the 12th edition, 23 awards were presented to companies for their exemplary performance in sustainability
Increasing Health Consciousness Among Consumers to Shift the Global Prebiotic Ingredients Market
Use of prebiotics in yogurt, beverages, and bakery items is expected to increase with rising consumer demand for fiber-fortified products, finds Frost & Sullivan
AqVerium – World’s 1st Digital Water Bank partners with Frost & Sullivan, UK to develop the world’s 1st “Blue Taxonomy” for collective global Water Stewardship
World’s First Digital Water Bank Teams Up with Frost & Sullivan UK to Introduce “Blue Taxonomy”
Technology Convergence Driving the Development of Sustainable Crop Protection Solutions
Governments’ strategies such as proposing reducing fertilizer losses by at least 50% by 2030 drives innovation in the sustainable agrochemicals market
Global Modular Construction Market to Witness Growth as Demand for Efficient Construction Methods Rises
A shifting awareness of the use of modular buildings and the response to new socioeconomic trends have unlocked growth opportunities in the MC market
Top 50 Start-ups Bringing Innovation to the Global Homes and Buildings Industry
The homes and buildings industry is moving toward decarbonization and digitalization solutions, expecting $50.99 billion in revenue by 2028, says Frost & Sullivan
Indian WWW Treatment Market to Experience a Boom as the Country witnesses increase in Private Investments
The government implements new business models to attract private market participants in the industry and expedite its growth, says Frost & Sullivan.
Rising Industrialization and Sustainability: Why the Global Transformer Market is Growing
Distribution transformers will continue to dominate the overall transformer market growth opportunities due to rising urbanization and industrialization, says Frost & Sullivan.
Green Ammonia Market Looks Optimistic as Efforts to Boost Clean Energy Rise
The strict compliance to lower greenhouse gas emissions drives green ammonia technology adoption among ammonia manufacturers, says Frost & Sullivan.
Lighting Controls Industry Expands as Awareness of Energy Solutions Grows
The need to replace conventional lighting systems promotes global lighting controls market growth, says Frost & Sullivan.
EU Sets 100% Reduction Target in Vehicle CO2 Emissions from 2035 Onward
The European Parliament has voted to set a 100% reduction target in vehicular CO2 emissions from 2035 onward, accelerating the shift from combustion engines to electric vehicles.
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Need for Infrastructure Resilience and Efficiency Fueling Demand for Digital Water Solutions, Finds Frost & Sullivan
Digital twins will experience high uptake due to their ability to optimize processes and enhance efficiencies
Transition to Net-Zero Emissions Catalyzes Asia-Pacific Utilities’ Adoption of Distributed Energy Resources
DER applications, including storage, solar, and EV charging infrastructure, will enable companies in the region to meet their sustainability goals, finds Frost & Sullivan
Global Oil & Gas Automation Market to See Positive Growth with Digitalization and New Disruptive Technologies
Spurred by technology innovation, the overall market revenue is expected to reach $24.6 billion by 2025, Finds Frost & Sullivan
Digitalization and Innovative Business Models Key to Transformational Growth in APAC, Finds Frost & Sullivan
With digital transformation and new technologies already disrupting multiple industries, organizations must innovate for the future to create value and drive better business outcomes.
Corporate Carbon-Neutral Strategies Set to Create New Revenue Streams for Companies
Development of new carbon regulatory frameworks will impact the global decarbonization goals and promote transparent messaging.
Frost & Sullivan Reveals Strategic Growth Opportunities Amidst Global Economic Recovery in 2022
Think Tank will explore how sustainability, digitalization and economic trends across regions are shaping regional and industry outlook
Top 10 Growth Opportunities in Energy & Environment Industry to Enhance Company Profitability
Frost & Sullivan experts present strategic insights on key trends such as the transition to a net zero future for carbon, hydrogen economy, and cognitive buildings and digital twins
Frost & Sullivan and TERI’s Sustainability 4.0 Awards 2021 Honor Companies Embedding Sustainability with Economic Value Creation
At the 12th edition, 23 awards were presented to companies for their exemplary performance in sustainability
Are You Leveraging the Drug Discovery and Early Development Outsourcing Services Ecosystem to Accelerate Growth?
By Frost & Sullivan
Organizational-level, Unified and Vendor-neutral Enterprise Imaging and Informatics Drive Effective Digital Transformation
Scalable enterprise-level solutions leverage all data at an enterprise level and can engage with the entire unified longitudinal patient record
Women’s Evolving Healthcare Issues Require Change in Mindset as well as Holistic and Personalized Care Delivery Models
The changing landscape of women healthcare presents 10 lucrative growth opportunities by 2030, says Frost & Sullivan
In Vitro Fertilization Services Industry to be Boosted by Innovative Technologies
Global IVF services market is expected to reach $43.97 billion by 2027, registering growth at a CAGR of 20.46%
Magnetic Resonance Imaging Market to Witness Growth Due to Increasing Demand for Scans
The prevalence of cardiovascular and oncology diseases and the shortage of radiologists have boosted the development of innovative MRI technologies
Cardiac Troponin Diagnostics Market Growth Boosted by High-sensitivity Point-of-care Testing
The rise in emergency department visits due to cardiac complaints drives the demand for cTn biomarkers
Medical Device Connectivity Market Growth Helps Overcome Healthcare Professionals’ Challenges
The MDC industry will witness growth due to the rise of MedTech and the incorporation of new technologies
Prenatal Genetic Testing Market to Improve Accuracy Through Technological Advances
Rising patient awareness drives technological advances and expands marketing for prenatal genetic tests globally, says Frost & Sullivan
How to Build Next-Generation Workplaces and Empower Employee Mental Health
World Mental Health Day creates an opportunity to raise awareness of mental health issues around the world and create strategies to support it in different workplaces
How Healthcare Organizations Can Enhance a Holistic Patient and Member View by Improving Insights from Data
A future-proof enterprise content management and enterprise imaging strategy across healthcare organizations enables individualized experiences, cost-saving efficiencies, and productivity gains
Frost & Sullivan Explores the Growing Impact of the Digital Front Door on Healthcare
Digital front door helps creating a new, consumer-centric paradigm in healthcare, at the same time it can improve patient satisfaction
Top Artificial Intelligence Trends Influencing the Future of Radiology
Discover how artificial intelligence will impact the radiology field, creating new growth opportunities
Technology Solutions to Boost Employee Experience and Improve Patient Outcomes
Workforce management solutions from DXC and SAP support healthcare providers in managing their teams while focusing on employees’ well-being and ability to deliver quality care.
How Can Digitalization of Medical Devices Boost Productivity in Med-tech
The digital transformation of medical devices encourages medtech players to deliver significant value for providers and patients, says Frost & Sullivan
Innovative Business Models in Digital Health Improve Efficiency Across the Care Continuum
Digital health solutions will become inevitable to support better healthcare outcomes at lower costs, says Frost & Sullivan.
Global Pharmaceutical Market Drives Innovative Digitalization to Accelerate Drug Discovery
According to Frost & Sullivan, digitalization across the pharmaceutical value chain will improve the industry efficiency.
Next-generation Diagnostics Paving the Way to Reach Personalized Medicine
Precision diagnostics will deliver personalized medicine to engage patients, says Frost & Sullivan
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
TOP STRATEGIC IMPERATIVES SHAPING THE MATERIALS INDUSTRY
By Frost & Sullivan
Are You Optimizing Your Growth Strategy to Leverage Key Opportunities in the Machines Industry?
By Frost & Sullivan
The Global Non-destructive Testing Software Market to Witness Growth with Improved Safety Requirements
The NDT testing software market is expected to reach $853.7 million by 2026, registering expansion at a CAGR of 11.1%
Digital Solutions and Sustainability Prompt the Global Building Management Systems Market Growth
Increasing industry convergence and the emergence of innovative technologies revolutionize the global BMS industry, says Frost & Sullivan.
Global Robot-based Metrology Boosted by the Need to Measure without Human Assistance
The global robot-based metrology market will reach $396.2 million by 2026
Global Metrology Software Market Driven by High Demand for Quality Control
Customer preferences shifting toward in-line metrology will be a key enabler for smarter metrology solutions and software, says Frost & Sullivan
Rising Internet Penetration and Falling Smartphone Prices Propel Indian Mobile Phone Market
The government’s Production-Linked Incentive scheme is helping manufacturing companies increase their year-on-year sales, finds Frost & Sullivan
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Frost & Sullivan Lauds Everbridge for its Innovative Leadership and Growth in the Command and Control Software Industry
Everbridge earns a top spot in the Frost Radar™: Command and Control Software for Critical National Infrastructure (CNI), Airports, and Safe Cities, Global, 2021
Connectivity and Advanced Technologies to Boost Growth Prospects for Global Semiconductor Devices
Increasing use of electronic content in automotive and factory automation is driving the semiconductor devices market globally, says Frost & Sullivan
Enterprises’ Need for OT Security Expertise Propels Growth of Industrial Cybersecurity Market
Revenues for buoyant global industrial cybersecurity market are likely to reach $10.2 billion by 2025, says Frost & Sullivan
Industrial Demand Creating Positive Outlook for Global Compressor Market
Major vendors and distributors must leverage new technologies such as IoT and real-time monitoring to gain a bigger market share in the global compressor market.
Computed and Direct Radiography to Boost Global X-ray Inspection Systems Market
Quality control and inspection solutions will be important to Industry 4.0-driven smart factories, creating growth opportunities.
Need for Shop Floor Metrology Solutions across Manufacturing Sectors Drives the Demand for Portable CMMs
Automotive and machine shops are expediting portable coordinate measuring machines market growth, finds Frost & Sullivan
Top 9 Growth Opportunities in the Industrials Market for 2022
Frost & Sullivan’s experts present strategic insights on key trends such as manufacturing 5.0, sustainability, and smart lifecycle services.
Automotive, Aerospace Industries Lead Demand for Stationary Coordinate Measuring Machine Market
CMM’s integration with mobile and robot-mounted systems for rapid measurement expedites global stationary CMM market growth, says Frost & Sullivan
5G Technology a Key Catalyst for Industry 4.0, Finds Frost & Sullivan
High bandwidth and low latency from 5G networks are driving process industries to partner with strategic 5G providers.
Industry-Driven Demand for Automation to Boost Operations and Productivity Spurs Global Machine Vision Market
APAC will remain largest and fastest-growing market for machine vision systems over the forecast period due to growing industrialization in the region, says Frost & Sullivan
Scaling Up AI Deployments: Harnessing Opportunities Sparked by Growing AI Maturity
By Frost & Sullivan
Companies to Action: Accelerating Digital Transformation with Disruptive Cloud Technologies
In the face of disruptive technologies and shifting business paradigms, being recognized as a “Companies to Action” marks a significant achievement, highlighting their ability to navigate complex landscapes and seize new opportunities with resilience and foresight.
Ascenty Awarded the 2023 Brazilian Company of the Year Award for Leading the Data Center Infrastructure across Latin America with Its Best-in-class Infrastructure
Ascenty is a trusted partner in customers’ digital transformation journey based on its extensive industry expertise, next-generation infrastructure, and outstanding services.
Gigamon Recognized by Frost & Sullivan for their Market-leading Position with the 2023 Global Company of the Year Award in Network Observability for Cybersecurity
Gigamon harnesses powerful network-derived intelligence and insights, enabling clients to enhance business agility, ensure cloud security, and minimize hybrid cloud cost and complexity.
Movers & Shakers Interview with Michal Harris, SVP Global Marketing, Beyond Now
Read our Movers & Shakers interview with Mei Lee Quah, Director, ICT Research, Frost & Sullivan and Michal Harris, SVP Global Marketing of Beyond Now
Movers & Shakers Interview with Shirin Esfandiari, Senior Director of Product Marketing, Oracle Communications
Read our Movers & Shakers interview with Mei Lee Quah, Director, ICT Research, Frost & Sullivan and Shirin Esfandiari, Senior Director of Product Marketing, Oracle Communications
Internet of Things (IoT): Top 10 Growth Opportunities for 2023
Geopolitical tensions, sustainability concerns, and supply chain disruptions are changing the IoT market perspective
Real-time Monitoring and the Ease of Data Retrieval Prompt the Adoption of Sensor Technologies
Security sensors integrated with other emerging sensor technologies provide safe and sophisticated security to critical assets
Leveraging Digital Technologies Can Help Grow Strong and Sustainable Communities
Capacity-building efforts provide the poor with sustainable tools that they can use to improve their circumstances and remove themselves from the poverty cycle
Green IoT and Communication Technologies Boost Environmental Sensor Market Growth
Sensors’ small size, low power, and communications capabilities will drive growth opportunities across industries
Identity for All Children and their Brighter Future in an Accessible Society for All
By utilizing biometrics, developing countries can effectively work with their citizens for wide-scale enrollment and expanded public service delivery
India 2023 Top 5 Trends to Watch in Information and Communication Technology (ICT) Industry
ICT has become one of the most significant growth catalysts for the Indian economy, contributing significantly to the country’s economic growth and welfare.
Strategic Hybrid Cloud in Enterprise IT to Improve Performance and Data Regulation
Businesses that implement strategic, well-managed hybrid or multi-cloud environments allow the flexibility of using the best cloud to optimize costs and reduce dependence on a single provider
Disruptive Times Call for Digital Agility
Transform Your Organization with the Right Communications Technology Partner
What Does Your Network Need to Boost Business Growth?
As more applications migrate to the cloud, a completely new connectivity architecture paradigm is being offered
New White Paper Sets Out Blueprint for Sustainable Operational Technology Security Programmes as Cyber Threats to Industrial Operations Rise
Frost & Sullivan and Applied Risk, a DNV company, have joined forces to publish a new white paper outlining practical steps for designing, implementing, and maintaining sustainable operational technology (OT) cyber security programmes.
Digital Technologies Drive Growth Across the Biomanufacturing Value Chain
Digitalization enables a sustainable, effective, cost-efficient, and error-free system for biomanufacturing.
The Powerful Legacy of TV in Today’s Streaming Platforms
The trend of streaming operators transforming streaming into a service with TV features is here to stay, whether it’s to broaden their sources of income, lower their pricing, or add more value to their services.
Frost & Sullivan Recognizes Future-Ready Companies at the India Manufacturing Excellence Awards 2022
19 awards were presented to companies for their exemplary performance at the 18th edition
Nominations for the 19th edition of the awards are open until May 15, 2023
Digital Technologies Drive Growth Across the Biomanufacturing Value Chain
Digitalization enables a sustainable, effective, cost-efficient, and error-free system for biomanufacturing.
Global Metrology Software Market Driven by High Demand for Quality Control
Customer preferences shifting toward in-line metrology will be a key enabler for smarter metrology solutions and software, says Frost & Sullivan
Robin Joffe Appointed as Partner-Managing Director of Frost & Sullivan Middle East, Africa, and South Asia
He brings over two decades of international business experience in market entry across various regions.
Frost & Sullivan and TERI to Recognize Indian Organizations Embedding Sustainability with Economic Value Creation at its Sustainability 4.0 Awards 2022
For the first time, Frost & Sullivan and TERI to bring in sustainable healthcare organizations under its India Sustainability 4.0 Awards umbrella
Corporate Carbon-Neutral Strategies Set to Create New Revenue Streams for Companies
Development of new carbon regulatory frameworks will impact the global decarbonization goals and promote transparent messaging.
India Manufacturing Excellence Awards 2022 will Identify and Recognize Future-Ready Factories
The 18th edition of Frost & Sullivan’s India Manufacturing Excellence Awards will take place on December 9, 2022.
Frost & Sullivan and TERI’s Sustainability 4.0 Awards 2021 Honor Companies Embedding Sustainability with Economic Value Creation
At the 12th edition, 23 awards were presented to companies for their exemplary performance in sustainability
Frost & Sullivan Recognizes Companies at the Forefront of Industry 4.0 Adoption at the India Manufacturing Excellence Awards 2021
The 17th edition of Frost & Sullivan’s India Manufacturing Excellence Awards honored companies at the forefront of adopting Industry 4.0. Organizations were evaluated on their manufacturing capability, extended supply chain reliability, and technology adoption.
Frost & Sullivan Reveals the Top 10 Global Economic Trends Shaping the Growth Prospects in 2021 and 2022
Discover how the 2022 economic growth outlook is set to evolve globally and potential factors impacting businesses in our upcoming growth opportunity briefing.
Frost & Sullivan Analyzes the Future of Digital Identity Management and Value Chain Compression
Join Frost & Sullivan’s webinar highlighting the impact of digital identity management and value chain compression on societies and industries on July 13th, 11 a.m. (EDT).
Seeq Lauded by Frost & Sullivan for Supporting Collaboration among Distributed Teams with Cloud-based Advanced Analytics
Seeq partners with cloud computing giants to deliver significant benefits to enterprises in era of remote working
Frost & Sullivan Commemorates Juneteenth as a Federal Holiday
The DEI Alliance at Frost & Sullivan honors the latest holiday addition with educational and celebratory resources.
Frost & Sullivan Announces New Vice President of Human Resources for Middle East, Africa and South Asia
She will be responsible for leading all human resources functions in the region and ensuring that the company continues to build a culture that attracts, engages, and develops the best teams.
Frost & Sullivan Unveils Strategic Opportunities in Digital Upskilling Shaping the Future of Work and Crowd Analytics
Various transformative trends and emerging opportunities are shaping the future of work and powering business and operational intelligence, opening up new avenues in digitalization
Frost & Sullivan Analyzes the Mega Trends Transforming the United States Through 2030
Millennials will remain the largest age group the US has ever seen (26.6% in 2030), ushering in a dramatic national shift as this population moves into leadership positions, family roles, and suburban living.
CEVA Lauded by Frost & Sullivan for Addressing the Challenges of Connected Devices with Its Smart Sensing MotionEngine™ Software
CEVA’s versatile and highly precise sensor fusion solution ensures an optimum customer experience in diverse consumer electronics and industrial markets
Manufacturers Employing Advanced 3D Metrology to Experience Greater Efficiencies across the Value Chain
Frost & Sullivan’s white paper shares how advanced metrology, specifically 3D metrology, enables optimized processes and performance.
Ecosystem Collaborations will be Pivotal if Kick Scooter Sharing Services Market is to Overcome Safety Concerns
Almost a year to date in April 2023, citing reckless driving, haphazard parking, and safety issues, nearly 90% of Parisiennes voted to ban e-scooter rentals in the city. By September 1, the 15,000 strong e-scooter fleet headlined by companies like Lime, Dott and Tier...
Are You Collaborating with the Shared Mobility Startup Ecosystem for Sustained Growth?
By Frost & Sullivan
Burgeoning Freight and Logistics Demands to Fuel Recovery of China’s Commercial Truck Industry
Truck electrification emerges as a major trend with electric powertrains set to reach 1 million units in 2030 on the back of technological improvements and government support. Commercial truck sales in China plunged from 3.7 million units in 2021 to 2.4 million units...
Automechanika and Frost & Sullivan Forge New Partnership for Global Impact
Frost & Sullivan, the global Growth Pipeline Company, proudly announces a significant milestone in its commitment to knowledge sharing and industry collaboration through a strategic knowledge partnership with the international Automechanika trade fair brand.
Chinese Automakers and Electromobility Trends Set to Shake Up ASEAN Passenger Vehicle Market
Competitive disruption will be evident across major markets like Indonesia, Malaysia, and Thailand as the process of transitioning to alternative powertrain solutions picks up pace and foreign companies like BYD, Great Wall Motor, LG, Hyundai and Tesla and BASF attempt to strengthen their presence.
In the Quest for Flexible, Efficient, and Sustainable Transport, Cities Turn to Technology-Driven Shared Mobility Modes
Strategic partnerships and Intelligent technologies will be crucial as a new shared mobility ecosystem takes shape.
Severing Connections: Implications of GM’s Decision to Embrace a Built-in, Native Operating System
Customers pushback over decision to abandon Android Auto and CarPlay, even as automaker looks to open up new monetization channels.
Demand for Smart City Solutions Spikes with Investments in Upgrading Telecommunication Networks
Smart city solution providers are developing new capabilities and products, expanding the ecosystem, says Frost & Sullivan
Global Demand for Autonomous Vehicles Encourages Alliances with Start-ups
Adoption of autonomous features will create new growth opportunities for start-ups, says Frost & Sullivan
Brazilian Connected Trucks Telematics Will Reach 2.59 Million Units by 2027
Carriers and insurance companies’ emphasis on risk management require telematics solutions on trucks that carry dangerous or expensive goods, says Frost & Sullivan
Engage with a Sustainable Future through Software-defined Vehicles at Frost & Sullivan’s Summit
In a two-day virtual summit, Frost & Sullivan dives into how software-defined vehicles and other key technologies will drive a clean energy future
Bankruptcy and One of the Largest Transactions Ever: the Future of SPAC Deals
Although Special Purpose Acquisition Company (SPAC) transactions have had their share of hits and misses, they continue to be instrumental in driving the electric vehicle market.
Electric Vehicles Lead the Way for Efficient Battery Solutions Globally
Electric vehicle manufacturers will adopt future chemistries over the next 5-7 years to overcome range anxiety and cost issues resulting in more effective battery solutions, says Frost & Sullivan
The Benefits of a Smart Connected Commerce for MSMEs in APAC
FedEx Ship More, Save More, and FedEx International Connect Plus Service (FICP) logistics solutions offer optimal logistics efficiency to MSMEs.
Need for Supply Chain Solutions Boosts Global Freight Visibility Demand
The global supply chain management platform market will likely reach $32.31 billion by 2026, predicts Frost & Sullivan
Global Independent Aftermarket Boosted by Out-of-warranty Tesla Vehicles
The increasing number of Tesla vehicles approaching the end of their manufacturer’s warranty coverage drives Tesla’s service-related revenues, says Frost & Sullivan
Ride share and Ride-hailing Boost Global Purpose-built Vehicle Demand
The purpose-built vehicle market improves vertical integration and brings customers and manufacturers closer by eliminating gaps, says Frost & Sullivan
How Digital Twins Can Save the Global Mobility Market from Expensive Errors
Digital twins can optimize decision-making, predict supply chain disruptions, and unlock new revenue streams, says Frost & Sullivan.
Evolution of the Smart Home Hub is the Next Growth Frontier, Finds Frost & Sullivan
Purposeful design and efficient use of space are transforming the home into a smart home hub
Frost & Sullivan’s Top 10 Trends for 2022: Metaverse and Cashless Economies to Drive Growth in Uncertain Times
Frost & Sullivan experts present strategic insights on key trends such as urban exodus, China’s technology crackdown, decentralized autonomous organization, and geopolitical instability
Mobile Medical Imaging Systems Improving Care Accessibility and Convenience for Patients
Portability and cost-effectiveness are key factors propelling the adoption of mobile medical imaging technology globally, says Frost & Sullivan
Rapid Expansion of 5G Network Rollout Drives the Global 5G Materials Market
Use of artificial intelligence technology in developing new 5G materials presents lucrative prospects for market players, says Frost & Sullivan
Frost & Sullivan Reveals How the Lights-Out Setting is Redefining Manufacturing
Companies have an opportunity to optimize their human capital and potentially save up to 20% of labor costs and generate a 30% increase in productivity output by switching to a lights-out operations model.
Frost & Sullivan Analyzes the Future of Digital Identity Management and Value Chain Compression
Join Frost & Sullivan’s webinar highlighting the impact of digital identity management and value chain compression on societies and industries on July 13th, 11 a.m. (EDT).
Frost & Sullivan Commemorates Juneteenth as a Federal Holiday
The DEI Alliance at Frost & Sullivan honors the latest holiday addition with educational and celebratory resources.
Digital Technology Advancements Propel Solar and Wind Farm Inspection Transformation
Leveraging digital technologies tp improve quality, safety, and productivity of the inspection says Frost & Sullivan
Frost & Sullivan Announces New Vice President of Human Resources for Middle East, Africa and South Asia
She will be responsible for leading all human resources functions in the region and ensuring that the company continues to build a culture that attracts, engages, and develops the best teams.
Energy Sector Illuminates with Technology Advancement and the Emergence of Prosumerism
Frost & Sullivan analysis notes distributed energy resources alongside smart analytics and energy storage to boost energy optimization.
Advanced Micro- and Nanofluidics to Revolutionize the Point-of-care Diagnostic Industry
Frost & Sullivan’s recent analysis, Advanced Micro- and Nanofluidics Revolutionizing the Point-of-care Diagnostic Industry, finds the scientific community has made tremendous progress in making microfluidics more autonomous with the integration of new powering mechanisms and sensor technologies, which can enable at-home or self-test devices.
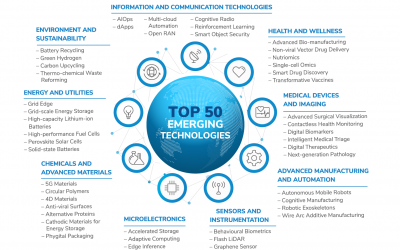
Frost & Sullivan Reveals the 50 Game-changing Technologies Transforming the Future
The webinar will highlight the top 50 technologies transforming the future and explore how these converging technologies will create unprecedented opportunities for new revenue models and innovative solutions that will transform the world.
Future of Hyperconnectivity Offers Billion-dollar Opportunities in the Connected Living Ecosystem
Frost & Sullivan’s recent analysis, Future of Connected Living, reveals that the device-to-person ratio will exceed 20 to 1 by 2030 as the world enters the era of hyperconnectivity and catapults organizations’ digital transformation.
Frost & Sullivan Unveils the Future of User Interfaces Shaping New Consumer Experiences
Frost & Sullivan’s recent analysis finds that user interface (UI) technologies have moved beyond the concept of simply representing machines to their users to enabling sophisticated and personalized interaction.
Frost & Sullivan Experts to Analyze Economic Outlook of a Post-pandemic 2021
Frost & Sullivan outlines top 2021 predictions and growth opportunities expected to transform the global economic landscape.
Freshwater Supply Challenge Stimulates Innovation in Reverse Osmosis Seawater Desalination Technology
Reverse Osmosis Seawater Desalination technology driven by rising water scarcity, widening water demand-supply gap, and demand for a reliable source of freshwater, says Frost & Sullivan
8 Global Shifts for 2021 Reshaping Industries, Governments and Society
Changes in user behavior patterns will trigger major changes in consumption and business models.
5G and Wi-Fi 6 to Disrupt Communication Protocols of Building Automation Systems
Frost & Sullivan’s recent analysis finds that 5G and Wi-Fi 6 will have a major impact on the connectivity of building technologies. 5G can provide greater accessibility when managing buildings more remotely and Wi-Fi 6 can provide faster data transfer speed between devices and enhance device performance at low energy utilization standards.
Frost & Sullivan Lauds Everbridge for its Innovative Leadership and Growth in the Command and Control Software Industry
Everbridge earns a top spot in the Frost Radar™: Command and Control Software for Critical National Infrastructure (CNI), Airports, and Safe Cities, Global, 2021
Improving Customer-facing Team Performance in the Hybrid Work Era
Productivity and efficiency will shape the era of hybrid working. Are your customer-facing teams equipped with the tools and data they need to reach their full potential?
Contract Development and Manufacturing Organizations Shifting Focus to Meet Future Demand
An integrated and agile approach enables the pharmaceutical CDMO industry to simplify manufacturing as it moves from primary care medicines to specialty medicines
How to Meet High Expectations as Customers Rapidly Shift to Self-Service
Customers’ increasing demands for self-service have pushed companies’ to reprioritize their digital roadmaps in a rush to meet customers’ expectations. Because live interactions cost companies 24 to 48 times as much as self‐service tools, improving operational efficiency by implementing self-service customer channels promises an enormous return on investment (ROI).
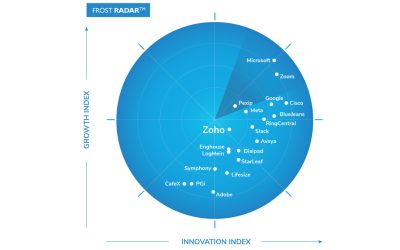
Frost & Sullivan Recognizes Zoho as a Growth and Innovation Leader in the Global Cloud Meetings and Team Collaboration Services Market
Zoho’s innovative solutions with recent technology improvements, increasing brand awareness, and smart collaboration services contribute to its market-leading position.
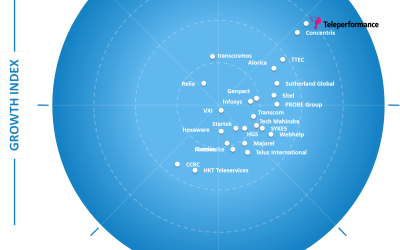
Frost & Sullivan Recognizes Teleperformance as Growth and Innovation Leader in the Asia-Pacific Customer Experience Outsourcing Services Market
Teleperformance continues to be a preferred partner for fast-growing and leading companies looking to redesign their CX and business processes
Red Hat Enables Energy Companies to Embrace Digital Transformation and Smart Partnerships to Adjust to Dynamic Changes
Utilities, O&G, and other energy companies adopt new technologies, capabilities, partnerships, and priorities to keep up with tectonic changes in the industry
Enterprises Adopting Intelligent Service Management Tools to Improve Employee Satisfaction
Businesses need to deploy a modern, intuitive ITSM platform to achieve faster time to value, finds Frost & Sullivan
Frost & Sullivan Recognizes MCM Telecom as a Leader in Mexico and Latin America in the Unified Communications as a Service Corporate Sector
MCM Telecom stands out for its wide range of telecommunications services that significantly increase the connectivity and productivity strategies for companies with hybrid work environments.
Frost & Sullivan Reconoce a MCM Telecom como líder en México y Latinoamérica en Comunicaciones Unificadas como Servicio para el Sector Corporativo
MCM Telecom destaca por su amplia oferta de servicios de telecomunicaciones que aumenta significativamente la conectividad y productividad de las empresas en ambientes híbridos
Leveraging the Cloud Helps Insurance Service Providers Optimize Business Processes and Improve Customer Experience
Service providers that support a variety of virtualization options can help companies become future-ready, finds Frost & Sullivan
Increasing Focus on Advancing Digital Customer Experience (CX) Transformation for Brands Boosts European Outsourcing Industry
CX outsourcers can positively affect the customer journey, helping brands deliver a consistent and appropriate digital experience
Advanced Performance Management Platforms will Enable Contact Centers to Deliver Enhanced CX
Incentivizing and engaging agents results in big leaps in performance and a subsequent rise in customer satisfaction, finds Frost & Sullivan
Retailers Offer Effective, Differentiated Customer Experience with Contact Center-as-a-Service
Digital solutions that offer deep visibility into customer journeys and supply chain ecosystems to drive superior efficiencies, finds Frost & Sullivan
Flawless Audio Boosts Effective Collaboration and Equal Opportunity in Hybrid Work
Frost & Sullivan and Shure’s latest white paper reveals that teams are less accepting of audio disruptions that derail their meeting agenda, make them less productive, or appear unprofessional.
Tailored Digital Platforms Help Banks Enhance the Customer Experience throughout the Customer Journey
Banks use technology partners to spread risks while raising customer service levels, finds Frost & Sullivan
Healthcare Providers to Gain a Holistic View of the Patient by Employing an Integrated Content Services Platform
A unified enterprise strategy that includes both clinical content and medical imaging is critical for delivering efficient and quality care, finds Frost & Sullivan
Aggressive Cyber Threats Drives Organizations to Adopt Extended Detection and Response Solutions
Extended detection and response (XDR) solutions are effective for adapting to a constantly evolving threat landscape.